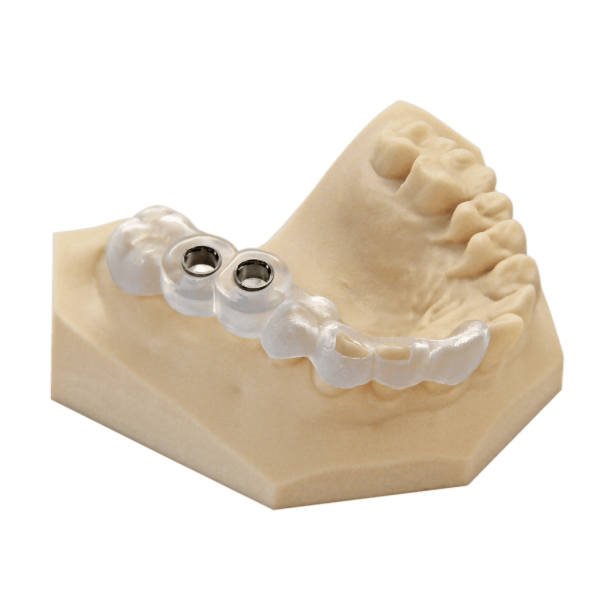


The EnvisionTEC E-Guide Resin is a biocompatible Class I material. It was developed for the production of high precision surgical drill guides that are used for implant surgery.
Free lifetime remote support on all products

Ask us about our discounts for education
The EnvisionTEC E-Guide Resin is a biocompatible Class I material, developed for the production of high precision surgical drill guides, used for implant surgery. The E-Guide is superior to traditional methods of manufacturing of implants during surgery. Drill sleeves can be inserted directly after printing.
The EnvisionTEC E-Guide Resin is registered with the FDA as a Class I medical device. The cost of high quality drill guides can be dramatically lower when produced using the E-Guide resin.
Applications: Dental
| Standard or Method | Description | Value |
|---|---|---|
| DIN EN ISO 20795-2:2013 | Flexural Strength | 79 - 85 MPa |
| DIN EN ISO 20795-2:2013 | Flexural Modulus | 2059 - 2030 MPa |
| DIN EN ISO 20795-2:2013 | Water Sorption | 30 - 23 μm/mm3 |
| DIN EN ISO 20795-2:2013 | Water Solubility | 0.5 μm/mm3 |
| Brookfield | Viscosity | 230 - 330 cP at 30° C |
| ISO 10993-5 | Cytotoxicity | Passed |
| ISO 10993-10 | Irritation and Skin Sensitisation | Passed |